Martin Berducci, MD1, Pablo Omelanczuk, MD1, Hans F Fuchs, MD2, Ryan C Broderick, MD2, Cristina R Harnsberger, MD2, Joshua Langert, MD2, Jorge Nefa, MD1, Pablo Jaureguiberry, MD1, Pablo Gomez, MD1, Laura Miranda, MD1, Garth R Jacobsen, MD2, Bryan J Sandler, MD2, Santiago Horgan, MD2. 1Hospital Italiano de Mendoza, Av. Lateral Acceso Este 1070- San Jose, Guaymallen-Mendoza, Argentina, 2Center for the Future of Surgery, University of California, San Diego
OBJECTIVE:
Single-incision minimally invasive surgery has previously been associated with incisions 2.0-3.0 cm in length and increased hernia rates. We present a novel single-incision surgical platform compatible for insertion through a standard 15-mm trocar we previously described in 6 patients with short term follow up data. The objective of this study is to evaluate the safety and feasibility of the platform in a larger collective and to present 1 year follow up data of our phase I trial.
METHODS:
The technology is currently a Phase II investigational device. It features a multiple-use introducer, accommodating the platform’s articulating surgical instruments, and is inserted through a standard 15-mm laparoscopic trocar. Cholecystectomy is performed through a 15-mm umbilical incision utilizing an additional epigastric 2-mm needle-port grasper for gallbladder retraction.
A prospective feasibility study was performed at a single-center. Inclusion criteria were age 18-75 years and biliary colic. Patients were excluded if they had acute cholecystitis, dilation of the biliary tree, severe coagulopathy, BMI > 40 kg/m2, or choledocholithiasis. Endpoints included the success rate of the platform, hospital length of stay, post-operative pain medication usage, cosmetic results, and presence of hernia. One year follow-up of phase I patients is also analyzed.
RESULTS:
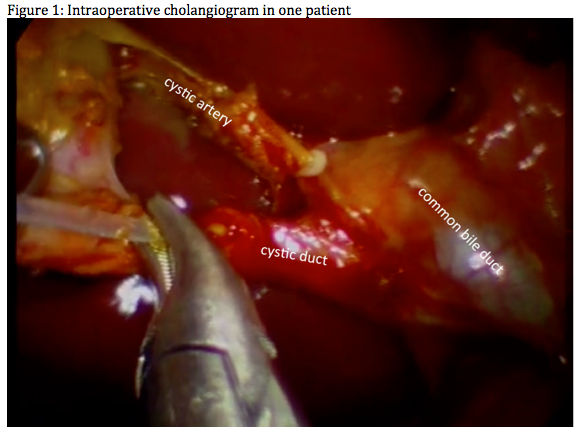
Twenty-four patients (21 females; phase I: 6 patients, phase II: 18 patients) with an average age of 41.7 years and BMI 26.6 kg/m2 underwent cholecystectomy with the platform. Average OR time was 76 min and umbilical incision length did not exceed 15 mm. Three cases were converted to standard laparoscopy. Initial difficulties with the clip applier were resolved in phase II. Two conversions were due to anatomical reasons. There were no intraoperative complications. Intraoperative cholangiogram was performed in one case (Figure1). Average OR time decreased from 91 min for the first 6 cases to 56 mins for the last 6 cases (Figure2). Post-operatively, 5 patients developed self-resolving umbilical ecchymoses, and 2 patients developed self-resolving seroma. Average length of stay was 7.8 h. Pain control was achieved with diclofenac for no more than 7 days. All 6 phase I patients had no adverse events nor evidence of umbilical hernia at 1 year follow-up. Evolution of the device is demonstrated in figures 3 and 4.
.png)
.png)
.png)
CONCLUSIONS:
This study demonstrates that single-incision cholecystectomy through a 15-mm trocar with the single-incision surgical platform is feasible, safe, and reproducible in a larger patient population. Long-term follow-up showed no hernias or other adverse events. Additional benefits include excellent triangulation and range of motion as well as exceptional cosmetic results. Further studies will be needed to evaluate longer-term hernia rates in a larger patient population.