Britney Harris MD, Nova Szoka MD
BaroNova received FDA approval for TransPyloric Shuttle (TPS) on April 16, 2019.
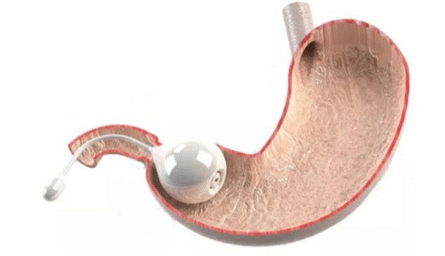
The TransPyloric Shuttle (TPS) is a minimally invasive treatment for obesity. The TPS system consists of a large and small bulb connected by a flexible silicone tether and an endoscopic delivery device. During delivery, the large bulb is distended with an internal coil and locked into the correct shape. Once the TPS is deployed endoscopically into the stomach, it causes faster filling times and delayed gastric emptying as peristalsis guides the small bulb into the small intestine and the large bulb to the pylorus. This device can remain in the stomach for 12 months, at which time it is retrieved endoscopically after removing the internal coil and collapsing the large bulb.
The randomized, double-blinded clinical trial ENDObesity II compared percent total body weight loss (%TBL) in patients treated with the TPS device versus a sham endoscopic procedure. Mean %TBL at 12 months for the TPS group was 9.5% compared to 2.8% in the control group (p<0.0001). A statistically significant reduction in insulin-resistance, blood pressure, and lipids was also observed in the TPS group.
The FDA determined this device has no prior substantially equivalent predicate devices.
Indications:
- Indicated in adult patients with a Body Mass Index (BMI) of 35.0 to 40.0 kg/m2 or a BMI of 30.0 to 34.9 kg/m2 with an associated medical condition, such as diabetes, who have failed medical weight loss strategies.
Contraindications:
- Prior surgery or endoscopic intervention that has altered esophageal, gastric, or duodenal anatomy
- Structural abnormality in the esophagus or pharynx such as a stricture or diverticulum that could impede passage of an overtube and/or an endoscope
- Esophageal abnormality such as erosive esophagitis, eosinophilic esophagitis varices, telangiectasia, or other anomalies that could cause bleeding or other procedural complications
- History of portal hypertension, cirrhosis, and/or esophageal varices
- Patulous gastroesophageal junction
- History of structural or functional disorders of the stomach including, gastroparesis, gastric ulcer, gastric mass, chronic gastritis, gastric varices, hiatal hernia (> 4cm), pyloric stricture
- Active gastric or duodenal ulcers
- Untreated Helicobacter pylori infection
- Inflammatory and other pathophysiological conditions of the gastrointestinal (GI) tract, such as Crohn’s disease
- Continuous therapy with known ulcerogenic medication (e.g., aspirin, NSAIDs)
- Coagulopathy or on anticoagulation or antiplatelet therapy
- Unable or unwilling to take proton pump inhibitors (PPI), or addition of PPI may cause adverse drug interaction with subject’s medication or interruption of treatment
- Diagnosis of bulimia nervosa or binge eating disorder or other severe psychiatric disorders
- Pregnancy or planned pregnancy in next 12 months
- Known or suspected allergy to any component materials in the device
- Any medical condition that would not permit elective endoscopy or anesthesia