Michelle Ganyo, MD, James Prieto, MD. Naval Medical Center San Diego
INTRODUCTION: Since the FDA’s approval of the LINX device in 2012 for the treatment of GERD, multiple studies have demonstrated the safety, efficacy, short and long-term outcomes of using this device. It has been well demonstrated to help with reducing the amount of reflux and gas-bloat symptoms and decreasing the use of PPIs all the while maintaining the ability of patient to belch or vomit. While there has been successful, long-term data with using the LINX device in the general population, there have been very few institutions that have performed this procedure on Active Duty population. The aim of this case series is to report the data from a single institution’s experience with placing the LINX device in Active Duty personnel for the management of GERD.
TOOLS & MATERIALS: From September 2016 to July 2017, 11 Active Duty personnel underwent the implantation of the LINX device for treatment of GERD. A retrospective review of their pre-operative history, operative data and post-operative course and symptoms were reviewed.
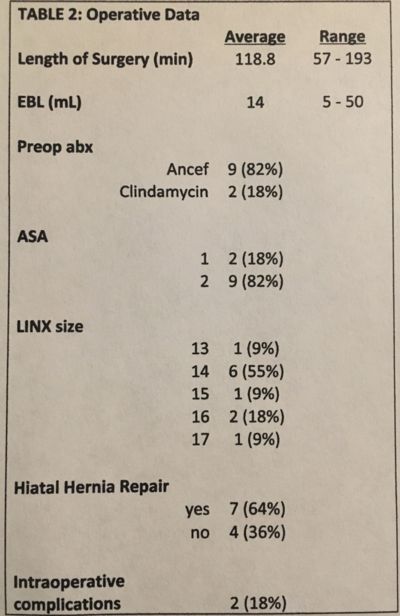
RESULTS: 11 Active Duty personnel underwent a laparoscopic placement of the LINX device for GERD. 2 patients had prior Nissen fundoplications, 4 patients had a hiatal hernia >3cm and 2 patients had > class A esophagitis. Patient demographics are listed under Table 1. The average operative time was 119 minutes, 64% of patient underwent a consecutive hiatal hernia repair and 2 patients had pleural violations that required a pigtail catheter during surgery, see Table 2. 1 patient required 2 returns to the OR for a recurrent hiatal hernia and migration of the LINX device. This patient ultimately underwent a Nissen fundoplication with collis gastroplasty. The average HRQL score improved from 27.3 to 4.8 and 85% of patients stated they were ‘satisfied’ with the procedure at 6-months post-operatively. The use of a single anti-acid medication for 0, 3 and 6 months were 64%, 0, 43%, and for double agents were 36%, 0, 14%. At the 6 month follow up, 43% of patients were not using any anti-acids to treat reflux, see Table 3.
CONCLUSIONS: Although our data is very limited due to a small population size and short follow up interval, the use of the LINX device to treat GERD appears to be a safe and effective option for Active Duty military personnel. We have also had reasonable outcomes for patients with >3cm hiatal hernias and more severe cases of esophagitis.
Presented at the SAGES 2017 Annual Meeting in Houston, TX.
Abstract ID: 86488
Program Number: MSSP09
Presentation Session: Military iPoster (Non CME)
Presentation Type: MSSPoster
.jpg?ssl=1)